Speed up your path to market with our cutting-edge IVRT services
Unlock Faster, Smarter Product Development with Acrux IVRT Services
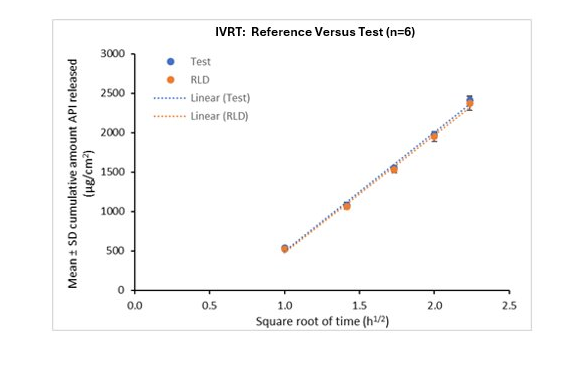
Acrux’s In Vitro Release Testing (IVRT) services offer a powerful, regulatory-aligned solution to accelerate your development pipeline and ensure product performance
- Experienced scientists with deep knowledge of IVRT
- Reliable data on drug release profiles
- Tailored IVRT methods for unique formulations and actives
- Regulatory-ready documentation
- Full analytical support, including method development and validation, protocols, test methods and associated reporting with adherence to ICH M10
- Ability to manufacture formulations if required
- Temperature and Humidity Controls
Pharmaceutical Applications
- Acrux have conducted successful IVRT studies approved by the US Food and Drug Administration (FDA)
- Bioequivalence studies conducted in compliance with FDA Draft Guidance for IVRT, USP <1724> and/or SUPAC-SS
- Our quality management system is in compliance with GLP and GMP requirements
- Successful outcome from IVRT inspection with no observations by US FDA, CDER (Centre for Drug Evaluation and Research), OSIS (Office of Study Integrity and Surveillance)